
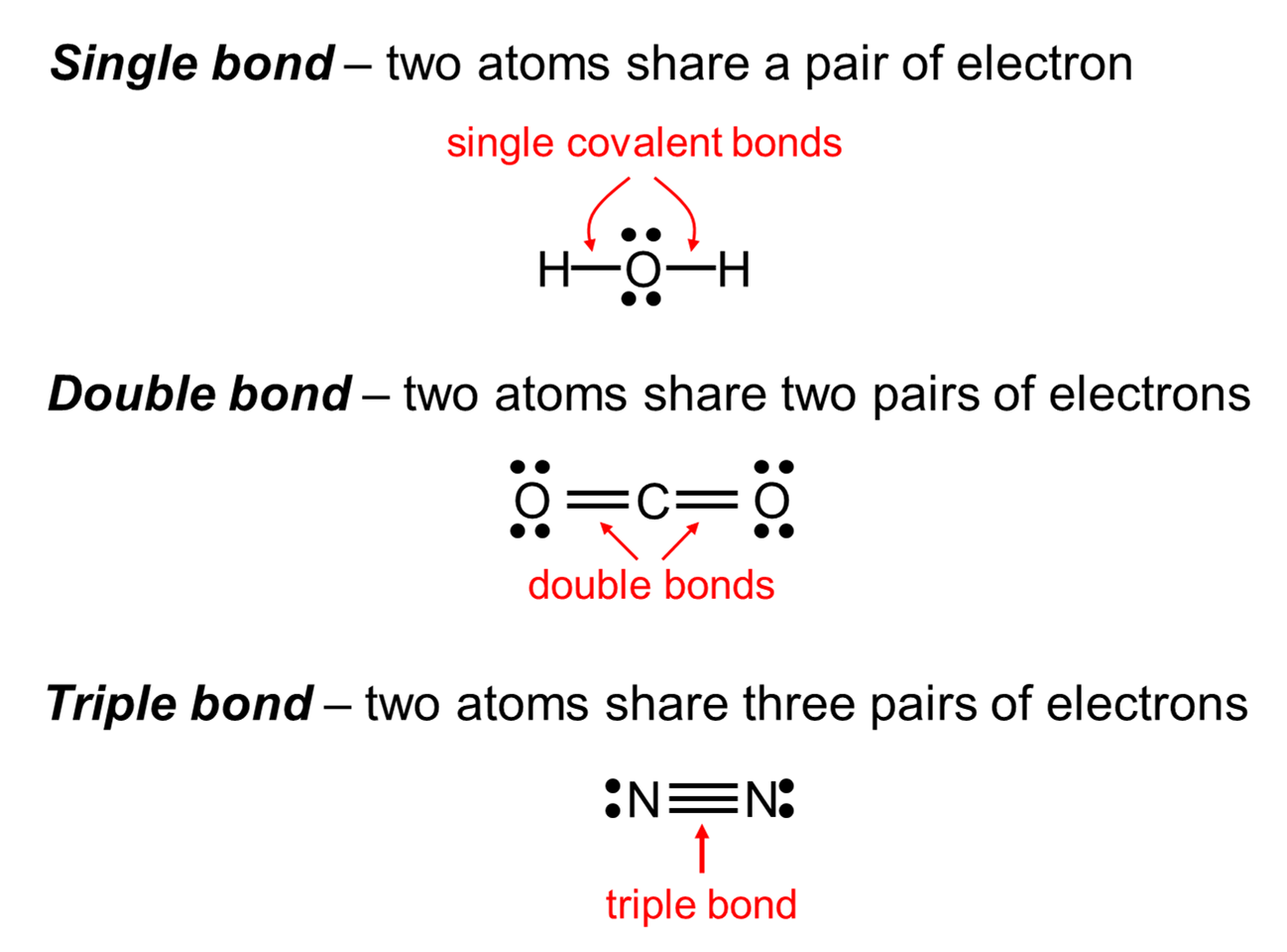
And so, um, in this case, we can see that we have a pie bond because we have a double bond, as well as a couple of single bonds between the hydrogen and the carbon, as well as the carbon between the carbon. So I think, is simply two carbons, double bonded that are saturated with hydrogen. And so, for our first example, let's look at how bonding occurs in a theme. And so when we talk about sigma and pi bonding, we'll be talking about the use of pie orbital's as well as hybridized orbital's. So again, this goes on access, and this is away from the access. And let's just draw a reference access to make this bit more clear. You can have an overlap between the orbital's and so you can create regions of bonding as well as anti bonding and So in this case, you would have two pies in this case you and have a single high. And so, if you're atomic, orbital's are lined up so that you can have bone overlap or, in other words, the same symmetry. And so this is how you would create a pi bon, and you can also do something similar, um, with the triple ones and so you can have de orbital's or to be orbital's overlap each other. And so these do not occur on the access, but actually outside of access. And so if we combine these or rolls together, you get a new orbital bombing and a new orbital of anti bonding. And so, for example, um, specifically for your pi bon, you can have a case where you have to be orbital's, and so you have an area of anti bonding and an area bonding.
#Sigma and pi bonds examples plus
A pie bond is one pie plus one single, and for a pie bond to occur, you need to have a signal interaction on a pie interaction. Let me just use the Greek symbols services.

And so, uh, this is often considered a double bond or a triple bond, where atrial wand is simply to pie plus one sigma. But essentially for a single bond, you combine two atomic orbital's to make a new orbital, and pie bonding is essentially a Sigma bond, plus a pie bond. And when we go into molecular orbital theory, this will make a bit more sense. If you overlap the oneness orbital's, you can have a new bonding orbital. And so an example of this is if you had 200 Jin's. And so, for a single bonds to occur, you need an aural that is aligned directly along and access for the overalls to overlap.

And so what happens is, if you have some kind of orbital, for example, and s Orbital and another ask for brutal thes or roles can overlap in such a way that you have a new orbital. So now that we've learned the hybridization of S P and D orbital's for molecules to make bonds, we can go back to a couple of examples to understand how Sigma and pi bonds for and so single bonding, which is designated by this great character, is essentially a head on bond.


 0 kommentar(er)
0 kommentar(er)
